Understanding Lithium extraction: DLE and traditional approaches
Understanding lithium extraction methods reveals what is at stake in the energy transition. Lithium is indispensable for the energy transition, powering Electric Vehicles batteries, grid-scale storage systems, and renewable energy infrastructure. LiCAN's Direct Lithium Extraction (DLE) technology and traditional approaches demonstrate how extraction choices impact our ability to build a sustainable future.
DLE transforms and speeds up lithium extraction for a sustainable future
DLE represents an advancement in lithium recovery that increases yield while reducing environmental footprint. LiCAN is implementing this process, using selective extraction techniques to address the growing demand for lithium in battery technologies and renewable energy applications.
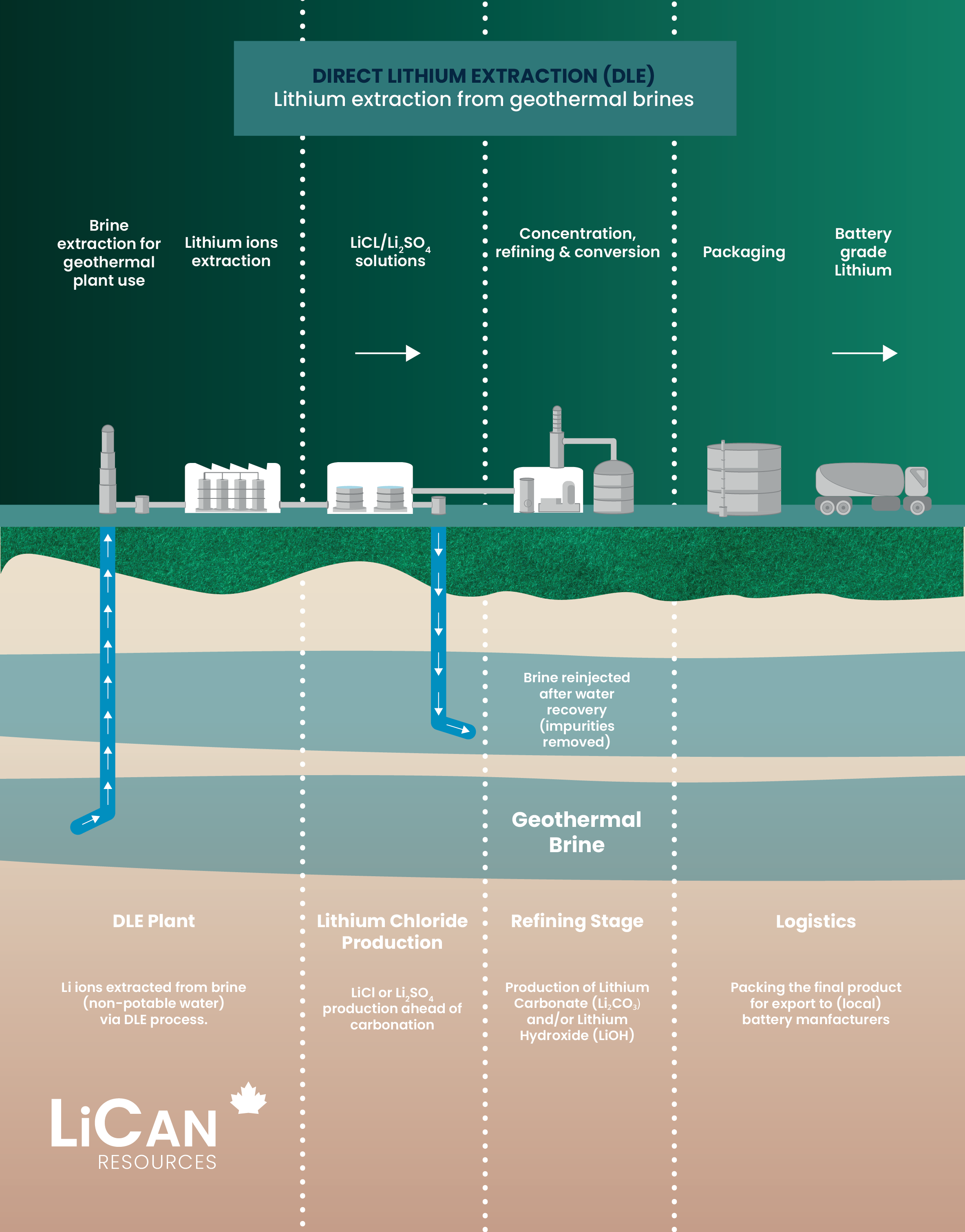
DLE process
DLE works by taking lithium-rich underground brine, extracting only the lithium, and reinjecting the water back in. The lithium is then processed into high-quality material for batteries.
Key advantages
DLE produces higher-purity lithium while consuming significantly less water and operating on much shorter timelines than conventional methods. This results in improved battery materials and streamlined production cycles.
Environmental
impact reduced
This method uses less land than mining or other brine-based methods. It consumes less water and produces fewer greenhouse gases, better preserving our environment.
Market efficiency
DLE recovers over 90% of lithium from brine in weeks instead of years compared to traditional methods. This enables faster, cheaper batteries while strengthening supply chain resilience.
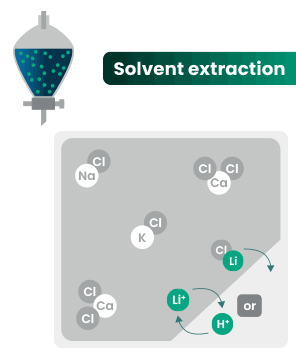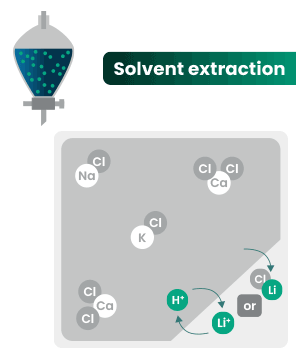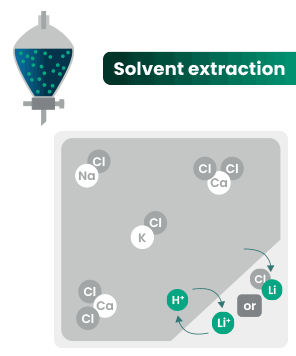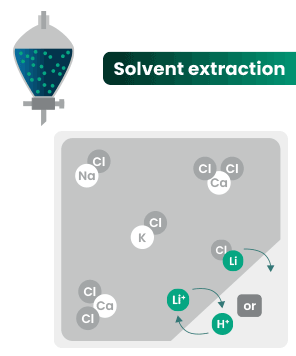
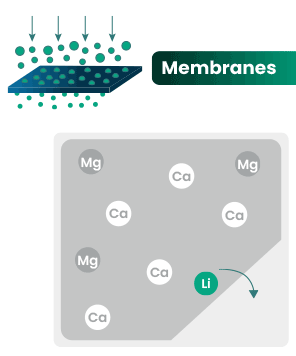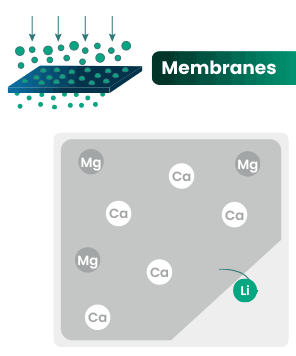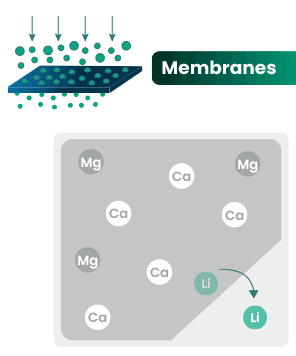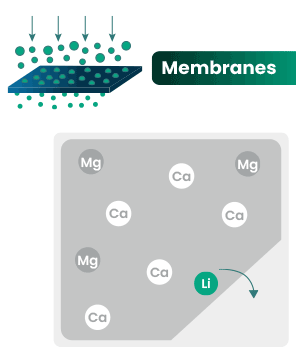
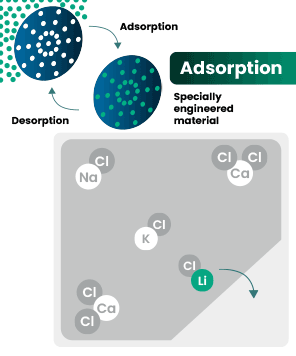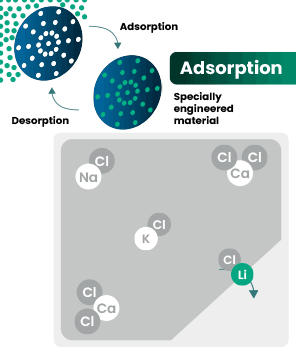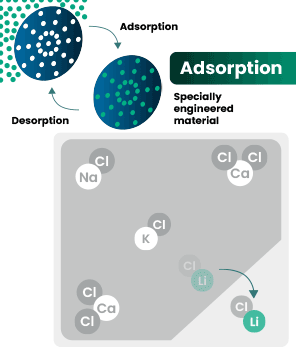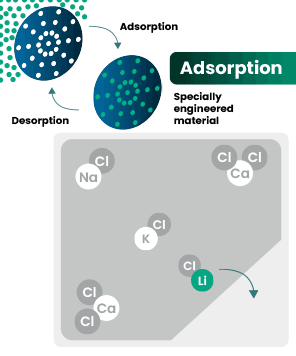
DLE captures lithium from brines
DLE uses different approaches to selectively remove lithium molecules from underground brine while leaving other elements behind.
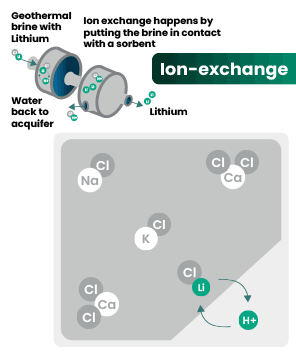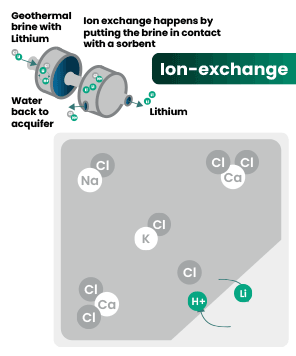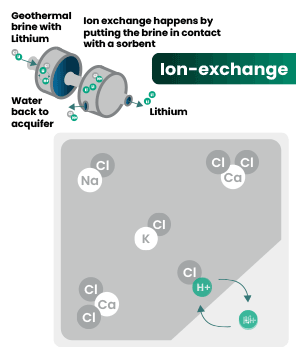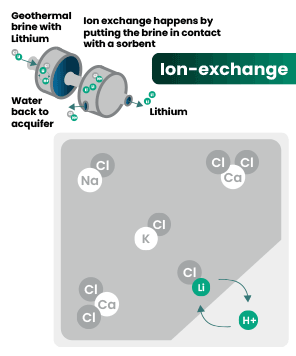
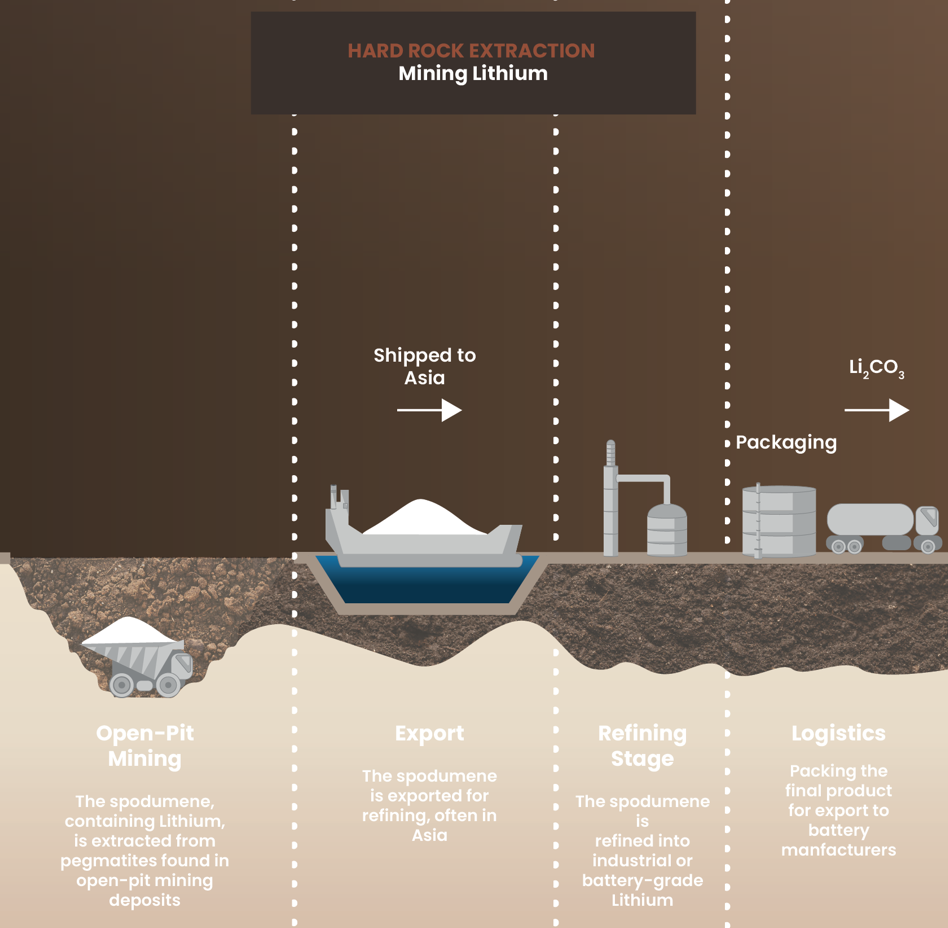
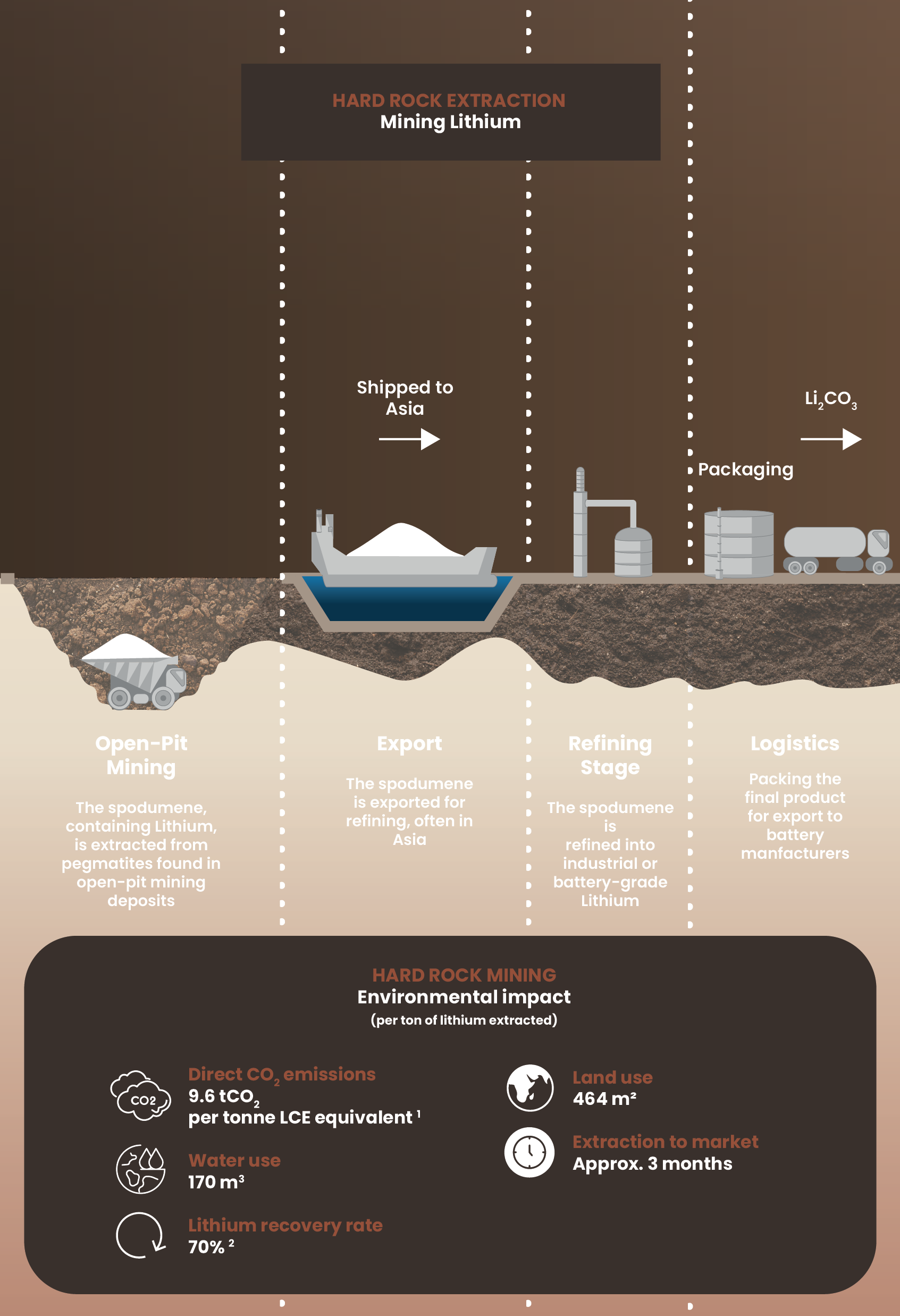
Hard rock mining:
High environmental cost for Lithium
Hard rock mining extracts lithium from crushed rock in open-pit mines. This process requires extensive digging, shipping the material overseas for processing, and multiple refining stages before the lithium reaches battery manufacturers. This approach takes approximately 3 months from mine to market.
Environmental footprint
This method produces emissions, requires significant water and land resources, and recovers only about 70% of available lithium.
The innovation imperative
Growing battery material demand highlights the need for alternatives that reduce emissions, save water, and improve recovery rates.
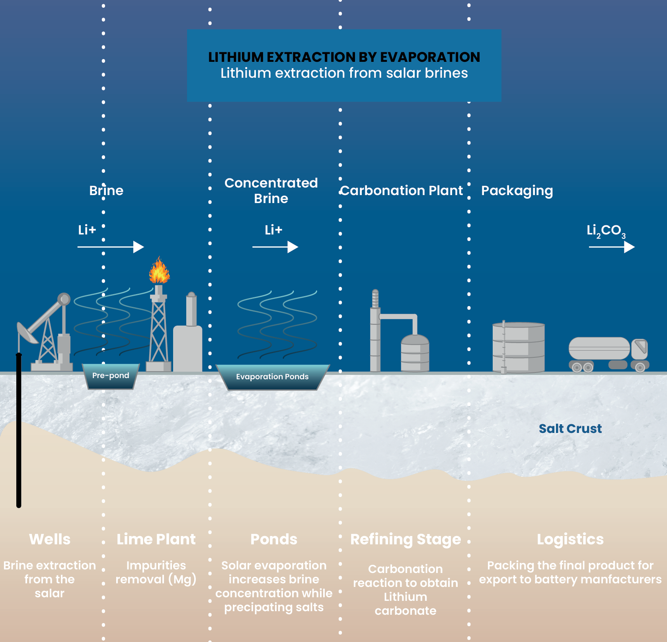
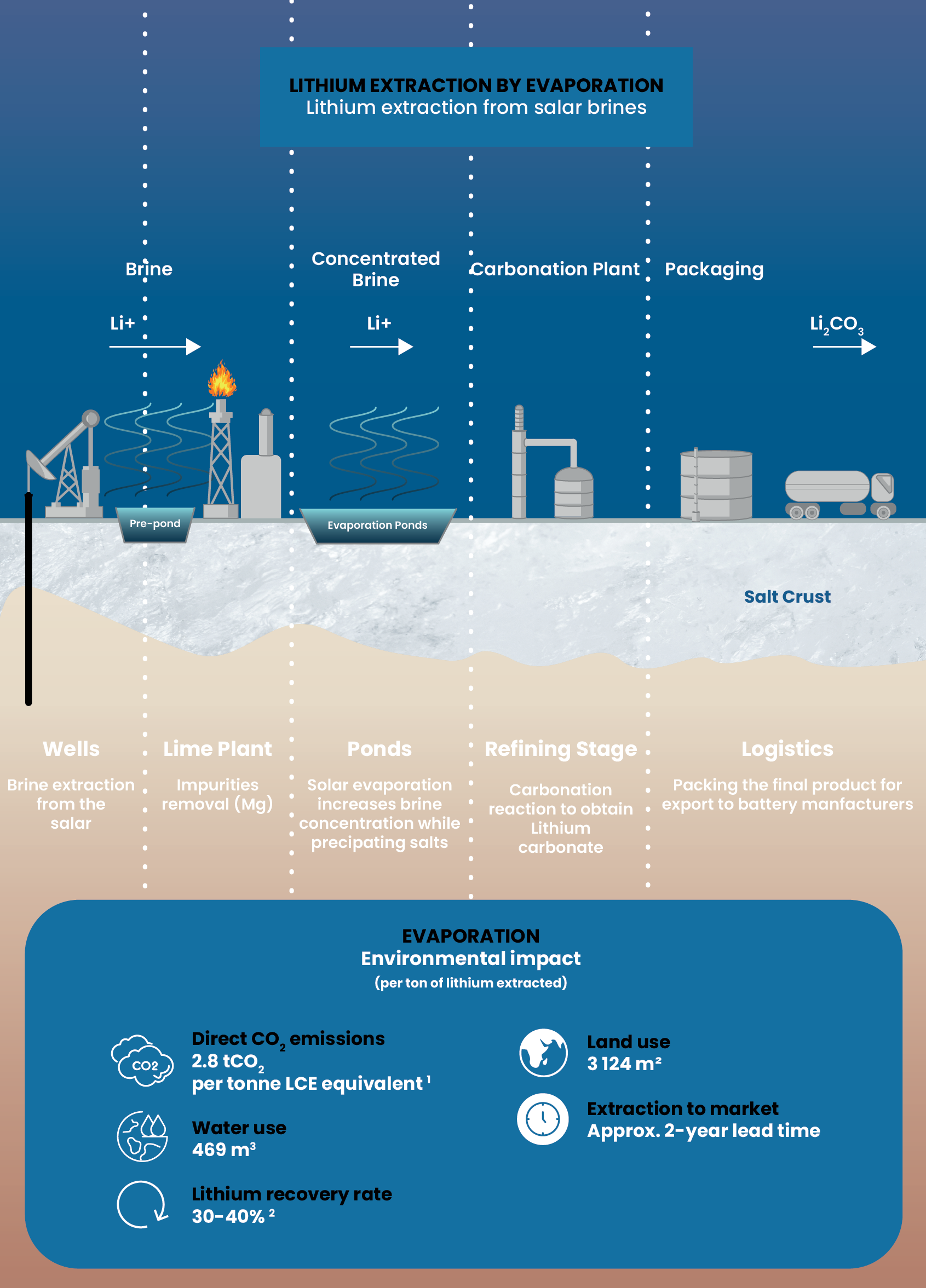
Lithium extraction by evaporation:
The traditional
brine-based method
The evaporation process extracts lithium from salt brines using a time-intensive natural approach. This method pumps lithium-rich brine into large evaporation ponds, also called salars, processes it through several stages, and eventually produces lithium carbonate after approximately two years.
Resource challenges
This method uses large amounts of water, requires extensive land for evaporation ponds, and recovers only about one-third of the available lithium, despite creating less pollution than mining.
The time factor
The two-year timeline from start to finished product creates long delays in lithium supply, making it difficult to respond quickly to the growing demand for batteries.
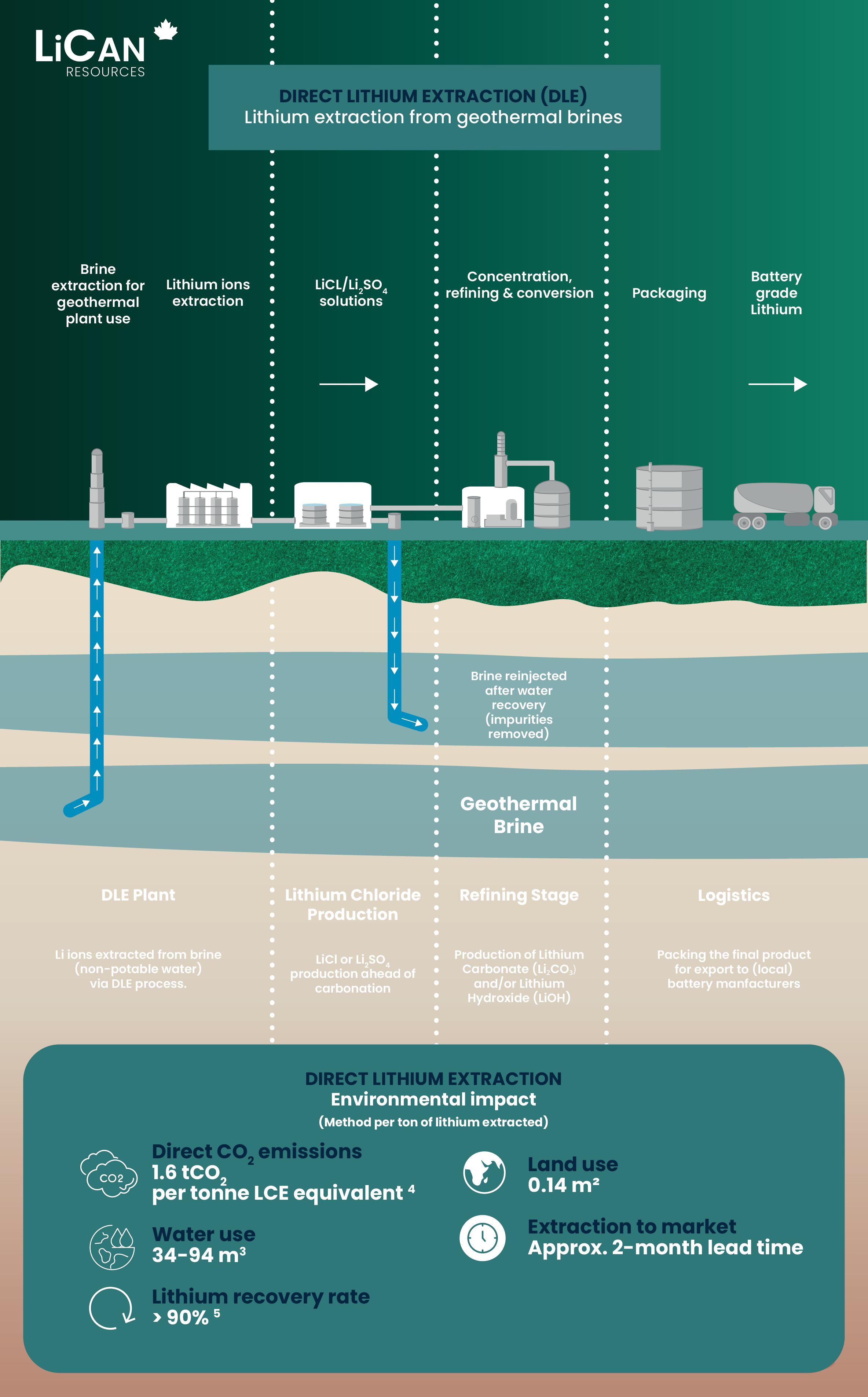
DLE in numbers
When compared to hard rock mining and evaporation ponds, DLE demonstrates superior performance across multiple key metrics, including CO2 emissions and water use.
(per tonne LCE equivalent)
lead time
lead time
Delivering smarter
Lithium production
With Direct Lithium Extraction technologies, LiCAN contributes to a cleaner, faster, and more sustainable future. Contact us today to learn how you can be part of the global energy solution.